Blog
Automate Your Notifications with Monitoring Automations! [Product Update 3.37]
In clinical trials, each patient inclusion or adverse event declaration represents a key moment that requires heightened attention. At EasyMedStat, we are committed to helping you optimize your processes by introducing a new feature: Monitoring Automations.
Why is this feature important?
Managing multiple clinical studies simultaneously can be challenging. In such a context, it’s easy to miss critical information, such as the inclusion of a new patient or the declaration of an adverse event. With our new feature, you can automate email notifications as soon as a patient is included or an adverse event is declared. This allows you to react quickly while avoiding human errors.
How does it work?
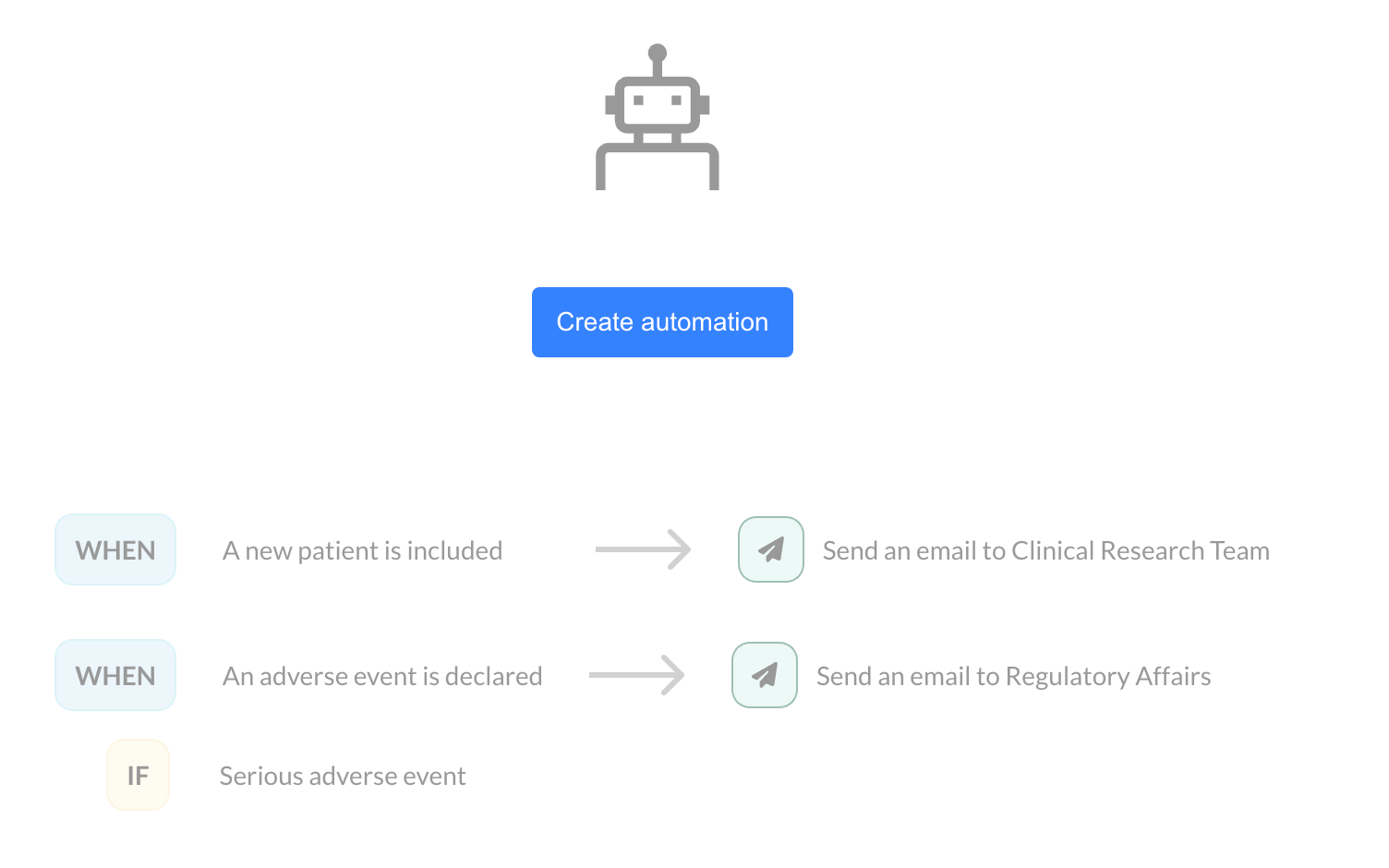
Monitoring Automation is based on a simple principle: you define an action that is automatically triggered by a specific event, such as patient inclusion or an adverse event declaration. Let’s look at two use cases:
Inclusion of a new patient:
When the inclusion of a new patient is validated in your eCRF, an email is automatically sent to the clinical research team. You can customize this message with variables related to the patient, the user, or the inclusion site, allowing the email to be contextualized according to your specific needs.
Adverse event declaration:
When an adverse event is declared, an email can be sent to the regulatory affairs team.
It is also possible to set a condition based on the severity of the event. For example, only serious adverse events can trigger a notification.
A valuable time-saver for your teams
Monitoring Automations offer incredible flexibility. Instead of constantly checking inclusions and events across your various studies manually, you can now rely on these automatic notifications to keep you informed in real time. This feature not only improves the responsiveness of your teams but also helps avoid oversight or delays in processing critical data.
Customization and flexibility
One of the main advantages of this feature lies in its customization. Whether it’s the email subject, content, or recipients, everything can be tailored to your needs. You can insert variables related to patients (such as inclusion date, patient ID) or users (first name, last name), ensuring that each notification contains relevant information.
Additionally, it is possible to configure notifications specific to one or more sites, depending on the setup of your study. For example, you can choose to receive notifications only for inclusions made at certain centers.
Conclusion
With this new feature, EasyMedStat takes another step forward in supporting clinical research teams. By automating essential tasks such as notifications for patient inclusion or adverse events, you enhance both the efficiency and safety of your trials. Don’t wait to explore and test Monitoring Automations in your EasyMedStat interface!
Stay connected, stay responsive, and focus on what matters most: the success of your clinical trials.
LATEST POSTS




New Features in EasyMedStat: Custom Record ID (CRID) and Test/Production Modes [Product Update 3.36]