Cutting-Edge Monitoring Tools for Your Studies
Accurate Statistical Analyses Start With Clean Data
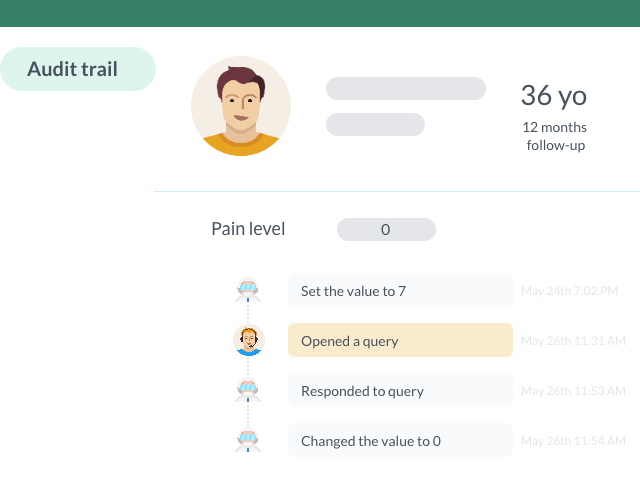
Easily Monitor Changes To Data with the Integrated Audit Trail
Every change to patient data is logged and visible to authorized users. You can keep track of your database with ease, ensuring your data is reliable for statistical analysis.
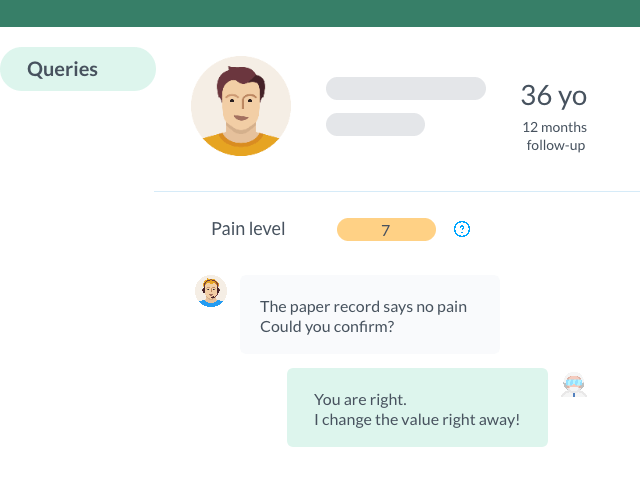
Control and Check the Quality and the Accuracy of Your Data with Queries
Open and assign queries to your study investigators to ensure the collection of quality in accordance with good clinical practices
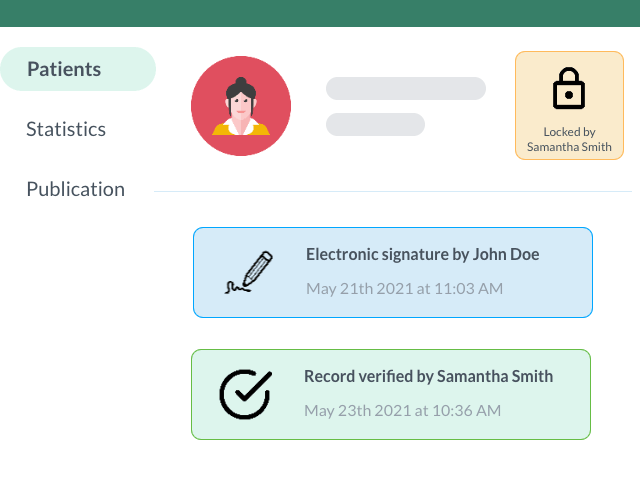
Increase the Quality and Security of your Research with Electronic Signatures
Sign, verify, and lock your patient forms to ensure remote source data verification with accurate data in accordance to the FDA title 21 CFR part 11.
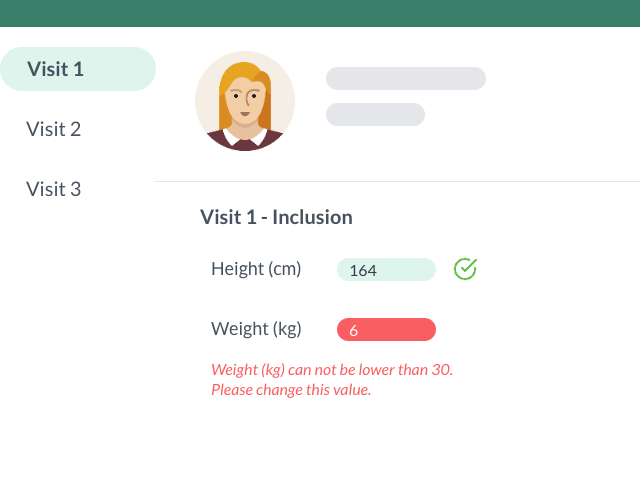
Decrease Monitoring Burden with Bound Values
You can set authorized values for your variables when creating your e-CRF to ensure that users will not enter incorrect data.
🚀 Let's start your next study!
Discover all the features of EasyMedStat with one of our experts.








