To conduct your clinical studies and investigations, the use of an e-CRF (Electronic Case Report Form) is becoming increasingly indispensable for efficiently collecting and managing patient data. In this article, we will dive into the details of e-CRF, its benefits for medical device manufacturers and academic researchers, as well as specificities related to regulatory compliance and the import of existing data.
What is an eCRF?
An eCRF is an electronic form used to collect, store and manage patient data within a clinical study. Unlike traditional paper methods, eCRFs allows online data collection, thus simplifying processes and offering numerous benefits.
What are the benefits of using an eCRF?
- Data Reliability: the use of eCRF improves the reliability of the collected data through built-in checks. Data validation features allow detection of errors and inconsistencies, thus reducing the risks of human errors. This contributes to obtaining more precise and better-quality data for more reliable analysis. This also implies a reduction in monitoring costs.
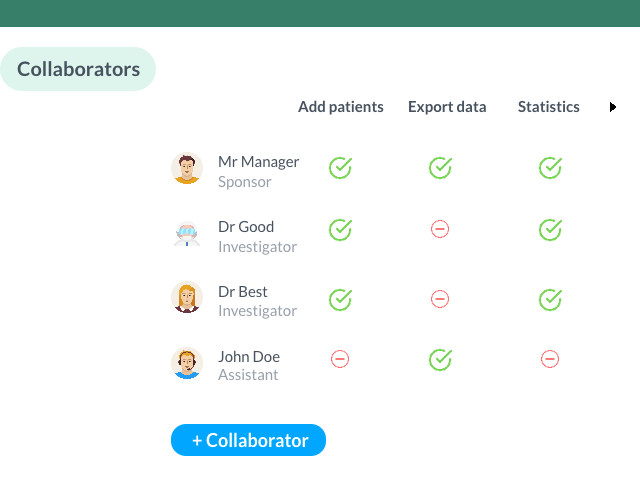
- Accessibility and Collaboration: an eCRF offers sharing and collaboration features that facilitate teamwork and allow better coordination among the various actors in a clinical study. Granular access rights allow defining user permissions, thus ensuring data confidentiality while allowing efficient collaboration.
- Data Security: eCRFs can ensure data security through advanced protection measures. eCRF platforms often use HDS (Health Data Host) servers which ensure secure storage of data in accordance with current regulations. This ensures the confidentiality and integrity of the collected data throughout the clinical study.
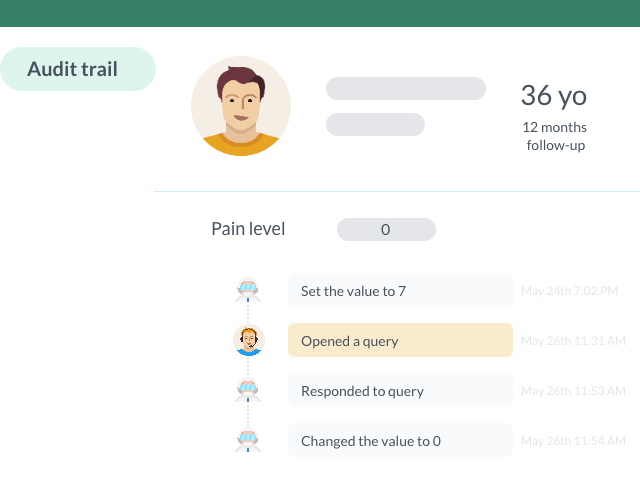
- Traceability: an eCRF allows full traceability of the actions performed on the data. The audit trail records all modifications, additions, and deletions made by users. This allows tracking and verifying each step of data collection, thus reinforcing the transparency and integrity of study results.
- Conducting decentralized studies: an eCRF facilitates conducting decentralized studies by reducing the need for on-site monitoring. Researchers can collect data directly from participants remotely, saving time and reducing travel-related costs. This opens new possibilities for conducting wider and more inclusive studies, reaching more diverse populations and facilitating patient participation.
Benefits of eCRFs for medical device manufacturers
- Regulatory Compliance: medical device manufacturers must comply with strict standards, such as ISO 14155, to ensure the quality and safety of their products. The use of eCRFs facilitates compliance by allowing structured data collection and improved traceability.
- Post-market studies (PMCF or SCAC):
medical device manufacturers are required to conduct post-market studies to monitor the effectiveness and safety of their products on the market. An eCRF simplifies the collection and analysis of data within these studies, thus allowing continuous monitoring and a rapid response to any potential issues.
- Physician Adherence: an eCRF offers a user-friendly interface for physicians participating in clinical studies. It facilitates data entry and patient monitoring, thereby improving their adherence and reducing entry errors. Additionally, by using an e-CRF in which statistical analysis is natively integrated, like EasyMedStat, your investigators can benefit from their data collection work. This also significantly improves their adherence and thus increases the chances of success of your clinical investigation!
Benefits of eCRFs for hospitals and academic researchers
- Ease of Implementation: the EasyMedStat eCRF is easy to use and implement, even for technology novice researchers. It does not require advanced programming skills and allows quick start of clinical studies.
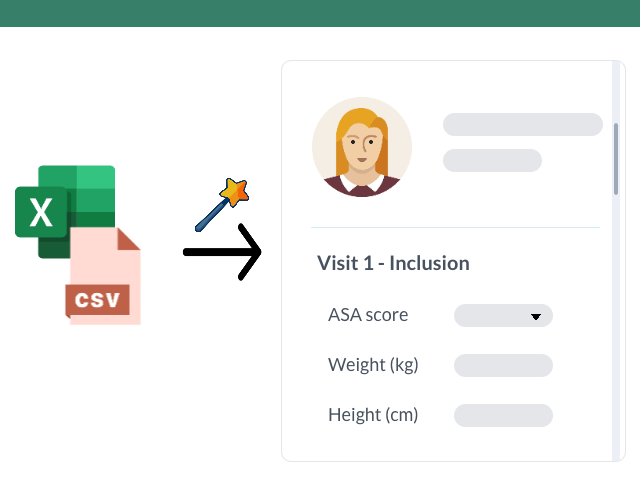
- Importing existing data: it is possible to easily import existing data from Excel files or databases, thus avoiding manual re-entry of information and associated errors. This allows transitioning from a spreadsheet-based collection to a more advanced collection via an eCRF.
- Real-time data collection: an eCRF allows real-time data collection, offering researchers the opportunity to track study progress, identify trends, and make informed decisions more quickly.
- Regulatory compliance and data traceability: eCRFs play an essential role in the regulatory compliance of clinical studies. It facilitates the generation of monitoring reports, data change management, and audit trail retention, thus ensuring full data traceability throughout the research process.
Training for optimal use of eCRFs
Adequate training is essential to fully benefit from the features of an eCRF and ensure efficient use of the platform. EasyMedStat offers two training options to meet users' needs:
- Online self-training module: EasyMedStat offers a self-training module freely accessible online on the
EasyMedStat academy. This module allows users to familiarize themselves with the features of the eCRF and learn to use them optimally. It provides educational resources such as explanatory videos, tutorials, and practical examples to guide users throughout the process. This online training allows users to learn at their own pace, depending on their needs and schedule. A certificate is issued at the end of your training.
- Video training sessions: we also offer video training sessions that allow users to master the creation of eCRF in just two hours. These training sessions are facilitated by EasyMedStat experts who guide participants through the steps of creating an eCRF, focusing on best practices and tips to optimize the use of the platform. Video training sessions offer an interactive learning opportunity where users can ask questions and get live assistance to resolve any issues or clarify specific concepts.
Integration service for personalized assistance
For users who prefer not to integrate their e-CRF themselves, EasyMedStat offers a dedicated integration service. Our team of eCRF experts can accompany you throughout the integration process, working closely with you to design and set up your eCRF according to your specific needs. This personalized integration service guarantees a smooth and efficient implementation of your clinical study, allowing you to focus on data collection and analysis without worrying about the technical aspect of e-CRF.
Conclusion
eCRFs have become an indispensable tool in the field of clinical studies, offering numerous benefits to medical device manufacturers and academic researchers. They allow efficient data collection, better regulatory compliance, increased traceability, and informed decision-making. For medical device manufacturers, an eCRF is a major advantage to comply with regulatory standards, while for academic researchers, it facilitates the accomplishment of high-quality studies while reducing errors and saving time. By adopting eCRFs, you can optimize your clinical studies, improve outcomes, and contribute to the advancement of medical knowledge.
Take control of your clinical studies now by using an e-CRF and discover the ease with which you can collect, manage, and analyze your research data.